McMaster experts answer your COVID-19 vaccine questions

McMaster nursing student Banan Bukhari administers a vaccine at FirstOntario Centre in Hamilton, Ontario.
News, social media, and daily conversations are filled to the brim with vaccine information and that can get pretty overwhelming. It’s okay to have questions you don’t know the answers to because information is changing so quickly.
To help address common questions, Faculty of Science communications coordinator Connor MacLean hosted an Instagram takeover last week, with the following McMaster experts providing answers:
- Zain Chagla, associate professor of medicine;
- Dawn Bowdish, professor of pathology and molecular medicine; and
- Matthew Miller, associate professor in the department of biochemistry and biomedical sciences
In case you missed it, here are some of the key takeaways from the session. To view the full takeover, visit our Instagram highlight reel capturing all responses.
Is it safe to mix and match vaccines and is there any concern about getting an mRNA vaccine for your second dose if you had an AstraZeneca first dose?

Dr. Bowdish: There’s no increased safety risk of mixing and matching different vaccine types. The risks are the same as for each, individual component. As well, emerging data suggests that mixing and matching certain vaccine combos works just as well, if not better, in inducing immune responses.
If you’ve had, as an example, AstraZeneca for your first shot – it’s perfectly safe and extremely effective to have an mRNA vaccine for your second shot. And, if you’ve had Pfizer for your first shot and you’ll have Moderna for your second shot, then that’s great too.
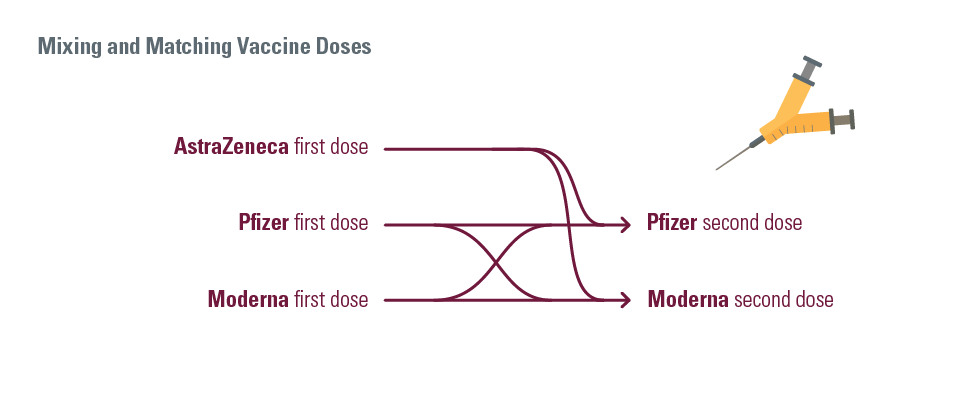
The vaccines are so similar that we really don’t believe there is any difference in having any mixing and matching. My advice to everyone is always get the first vaccine you’re offered as quickly as you’re offered so we can fight the Delta variant and get back to McMaster safely.
Should I be worried about getting infected after being vaccinated and does that mean the vaccines don’t work?
 Dr. Miller: Although COVID-19 vaccines have demonstrated really astounding efficacy, it’s of course the case that no vaccine is perfect. As a result of that, it’s expected that a certain number of breakthrough infections will occur, but this is nothing to get worried about and it doesn’t mean that the vaccines aren’t working.
Dr. Miller: Although COVID-19 vaccines have demonstrated really astounding efficacy, it’s of course the case that no vaccine is perfect. As a result of that, it’s expected that a certain number of breakthrough infections will occur, but this is nothing to get worried about and it doesn’t mean that the vaccines aren’t working.
Common reasons breakthrough infections occur include vaccination of individuals who have conditions or who are taking medications that prevent their body from mounting strong immune responses to the vaccine. This could include frail, elderly individuals, for example. Or individuals who are taking chemotherapeutics or immunosuppressive drugs.
In addition, some individuals will experience breakthrough infections even though they appear to be otherwise healthy. The good news is those infections tend to be much less severe than they would have been had they not received a vaccine.
It seems like COVID vaccines were approved really quickly. What was the testing and development process?
 Dr. Chagla: I want people to know that these vaccines actually have a large number of people in the trial – actually bigger than most vaccine trials out there. That really was the fact that these companies went through lengths to make sure people had access to vaccines.
Dr. Chagla: I want people to know that these vaccines actually have a large number of people in the trial – actually bigger than most vaccine trials out there. That really was the fact that these companies went through lengths to make sure people had access to vaccines.
These vaccines were done relatively quickly because COVID was circulating. Our prior vaccines, like shingles, take 5, 10, 20 years for things to happen. Whereas with these vaccines, unfortunately people were getting COVID and that’s the state of the world right now. They met their outcomes pretty quickly because people were getting COVID in both the placebo arms and the vaccine arms.
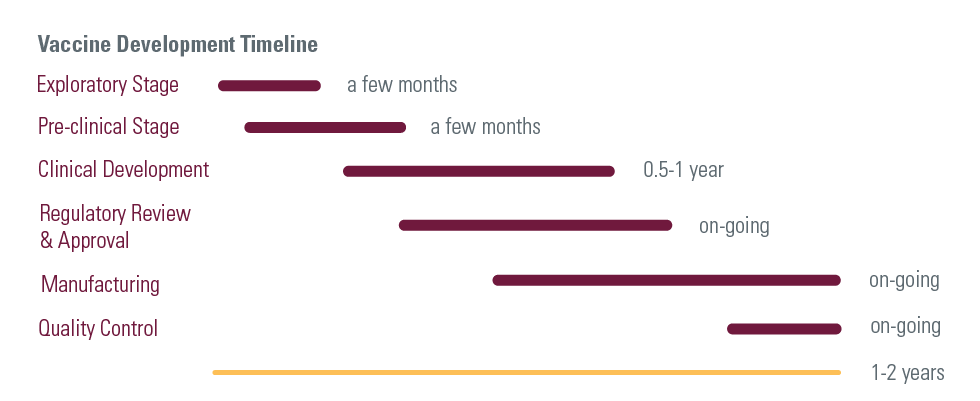
These clinical trials involve thousands of participants, where half receive a placebo and half receive the vaccine. This allows researchers to see how well the vaccine protected the study group compared to the controlled group who received the placebo.
Finally, from a regulatory standpoint, the major difference here is that because this was a global emergency, regulators took a look at the data at the same time the trials were being performed. Normally, trials wrap up and they are submitted to Health Canada. In this case, Health Canada was looking at the data as the trial was being performed. The same review of the data was ongoing, but it was done in sequence to minimize time and get this vaccine out there.
I want to reassure people that these vaccines work, and they were approved in a very similar process to prior vaccines.
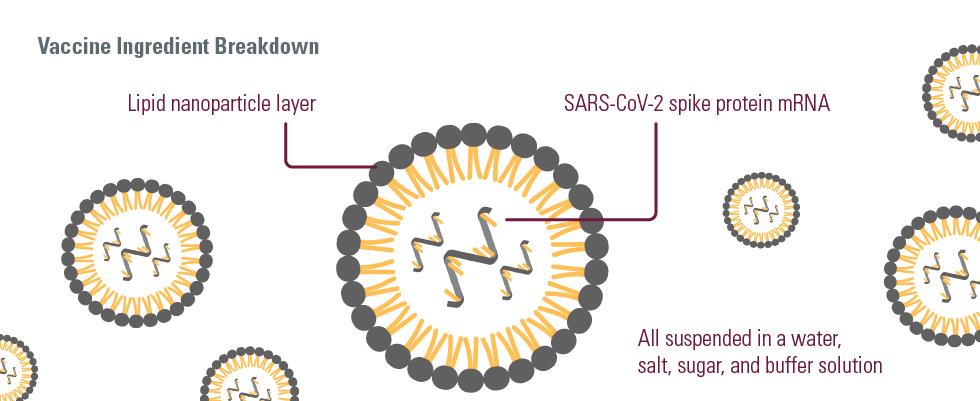
How do mRNA vaccines work?
 Dr. Miller: mRNA COVID-19 vaccines work by delivering the code to our cells that teach it how to make the spike protein of SARS-CoV-2.
Dr. Miller: mRNA COVID-19 vaccines work by delivering the code to our cells that teach it how to make the spike protein of SARS-CoV-2.
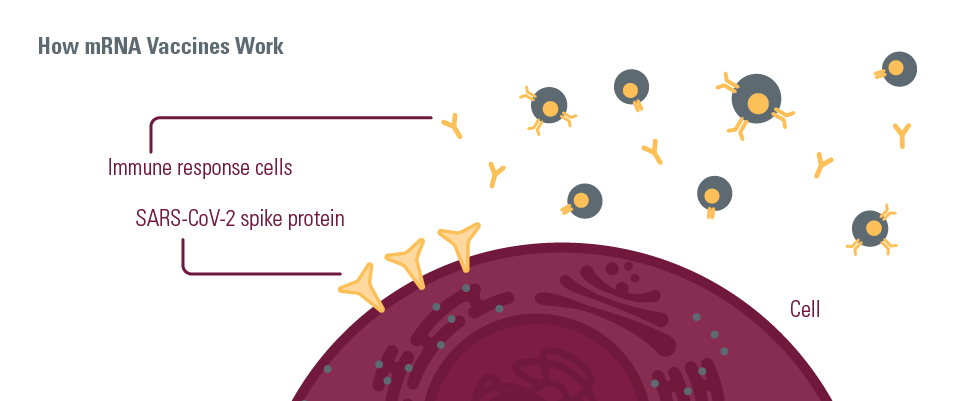
Spike is the protein found on the outside of the virus that helps it to bind to and enter our cells. When our cells make Spike, which on its own is an innocuous protein—it teaches our immune system to generate antibodies and t-cells.
In some ways, this serves almost like a wanted poster for criminals in that it alerts our immune system to what the real virus looks like. So, if we ever encounter it, our immune system is able to respond rapidly and strongly to prevent us from getting infected.
 The graphics above were developed in partnership with Dr. Chagla, Dr. Bowdish and Dr. Miller with additional resources from Science for Everyone, Health Canada, and Centers for Disease Control and Prevention.
The graphics above were developed in partnership with Dr. Chagla, Dr. Bowdish and Dr. Miller with additional resources from Science for Everyone, Health Canada, and Centers for Disease Control and Prevention.
—
For more information:
Stay tuned for more information on COVID-19 vaccines from McMaster’s immunology and infectious diseases experts. In the meantime, check out Student Wellness Centre’s web page to answer common vaccine-related questions.
Booking a vaccine appointment:
Click here for information on Hamilton vaccine procedures and frequently asked questions.
Book online here: Ontario Vaccine Booking Site (English)
Book over the phone: Call the Provincial Vaccine Information Line at 1-888-999-6488. If you don’t have a health card, call the Public Health Services COVID-19 Vaccine Hotline at 905-974-9848, option 7.


